EPDM
EPDM is a type of synthetic rubber based on ethylene propylene and a third monomer as well as a double bond that causes sulfur deposition.
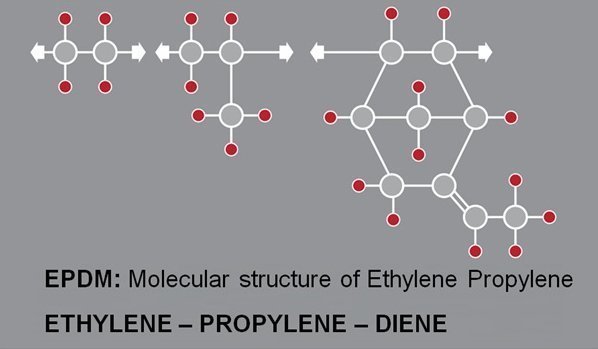
EDPM - Ethylene Propylene DN
EPDM is a type of synthetic rubber based on ethylene propylene and a third monomer as well as a double bond that causes sulfur deposition.
Due to its chemical structure, this material is resistant to water and steam, and it is also suitable for an environment with a high ozone percentage. For the reasons mentioned, EPDM is ideal in the following situations:
Water to boiling point
Steam
Hydraulic systems such as brake systems
Ozone
weathering
Alkaline substances
polar solvents at room temperature
The temperature range is between -50 and 150 degrees Celsius
code
EPDMW85A
hardship level
5 ± 88
Color
Black
Temperature range
-40 to 130
Description
Suitable for minerals
data sheet
Disadvantages of EPDM:
- Poor resistance to petroleum-based fuels and non-polar solvents
- Poor resistance in acidic environments
(EPDM) is a type of polymer that has many different applications in the industry. EPDM is a synthetic rubber that has high resistance, which stands for Ethylene Propylene Diene Monomerase. This material was first introduced in 1962 by Karl Ziegler. But this discovery was later expanded by the same German scientist and Italian scientist “Giulio Natta” and won the Nobel Prize. This substance was used and welcomed in the United States in these years because petroleum derivatives were very expensive.EPDM was able to find its place in the market due to the high cost of isogum or bitumen and was widely used in construction. Today, this material is used in sealants, gaskets and other rubber products due to its insulation against environmental factors. Today, EPDM is known as one of the most widely used materials in the world, and the tires produced from this material are the most widely used rubbers. are offered in the market.
Analysis of EPDM
The amount of ethylene in the polymer chain is about 60 to 80 percent. Commercial types of these tires have 55 to 75% synthetic ethylene, which can be used to create different types of elastomers. It is interesting to know that the main role of propylene in this copolymer material is to disrupt the polyethylene chain order and cause it to fail and prevent the crystallization of ethylene, thus enabling the sulfur curing of this compound. If the hydrogenation on the rubber chain is done in a natural way, the copolymer is obtained as a mixture of ethylene-propylene rubber, and since the main chain of the same copolymer is saturated, it does not create the possibility of sulfur line.
In order to provide the possibility of curing, the third monomer should be placed on the side of the main chain and added to its molecular structure.
In general, it can be said that this copolymer is not linear, although the degree of branching of this material depends on the percentage of monomers and the position of polymerization. More branching occurs if the percentage of the third monomer increases. It is better to say that the analysis of EPDM helps to better use it in different parts. Another noteworthy point is that this material maintains its insulating ability inside the cables up to kv60 and does not lose its rubber ability. For this reason, it is used as wire and cable covers in various productions.
.
